The Case of One Baby, “Three Parents”
Image credits: Luma Pimentel | Unsplash
In a relatively quiet but history-making development, at least eight children have been born in the UK using a reproductive technique that combines the DNA of three people. The goal here was to prevent the transmission of devastating mitochondrial diseases from mother to child. While this isn’t the first time that a “three-parent baby” has been in the news, here regulatory oversight in the UK led to an ethically rigorous process with extensive monitoring.
How it Works
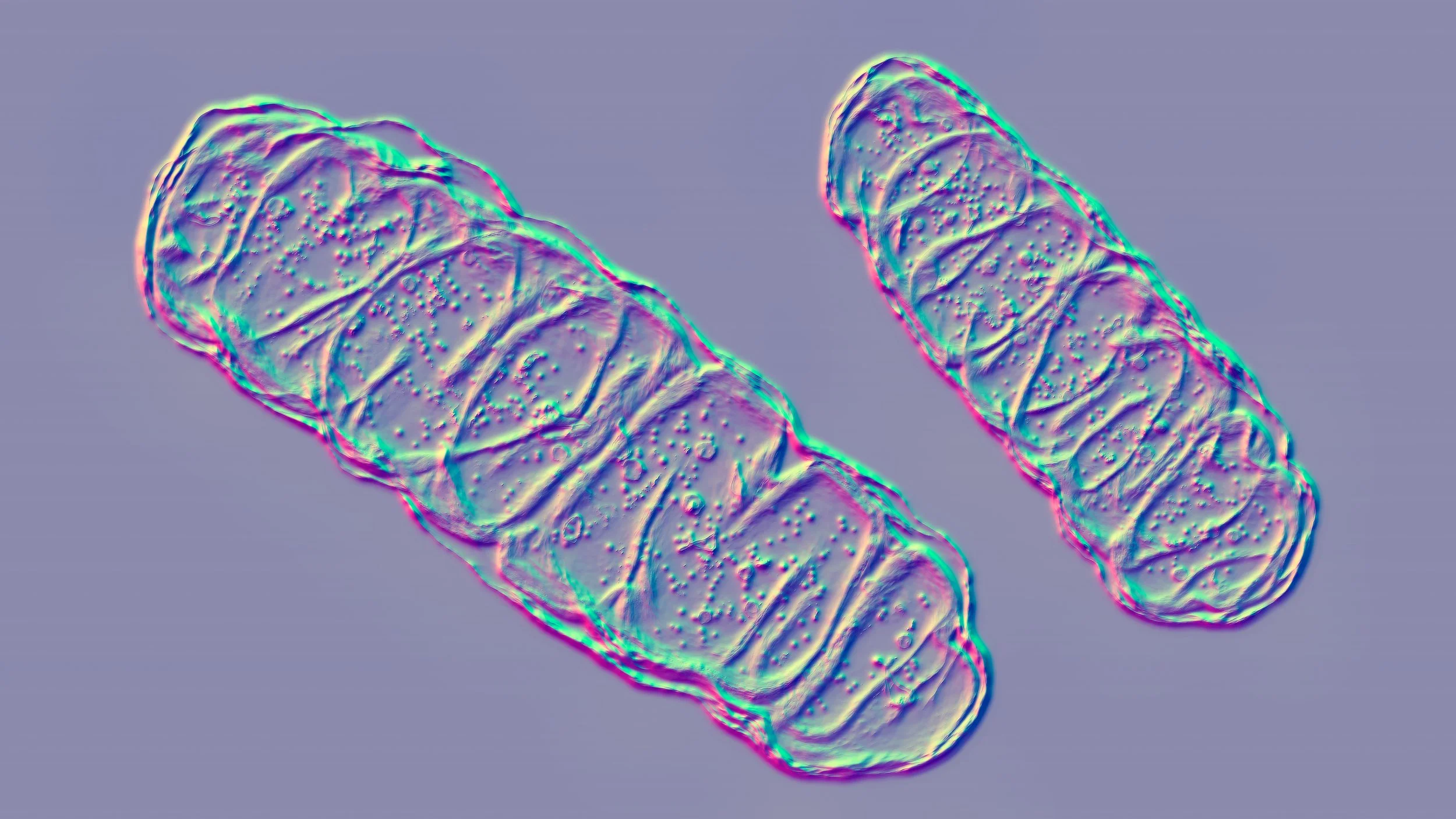
Here’s how it works. Mitochondria (the tiny powerhouses inside our cells) carry their own DNA, separate from the 20,000+ genes we usually think of as “our DNA”, which is found in each cell’s nucleus. Why? According to a broadly accepted scientific theory (by which I mean an overarching framework that explains and predicts lots of things, not a “guess”) called the Endosymbiontic Theory, at some point in our deep evolutionary past, a free-living bacterial cell was engulfed by a larger cell … and took up permanent residence inside, bringing along its own genome. The descendants of those once-independent bacteria can be found in today’s animal cells (as mitochondria) and plant cells (as mitochondria and chloroplasts).
An image of a mitochondria | Image credits: Dr_Microbe | Adobe Stock
During human reproduction, the egg is the source of mitochondria for the fetus, meaning a child almost always inherits its mother’s mitochondria. If the mother’s mitochondria carry a DNA mutation that causes a disease, then the child is very, very likely to inherit the same disease. I’m being careful with my language here because biology never fails to surprise me, but in general, each of us has inherited our mother’s mitochondria and all the DNA that they carry.
To avoid this, doctors and researchers at Newcastle University used a technique called mitochondrial replacement therapy. They fertilized the egg of a mother with mitochondrial disease with sperm from the father, then removed the combined nuclear DNA (leaving the faulty mitochondria in that egg). From the donor egg, the doctors removed the donor’s nuclear DNA but kept the mitochondria (which do not have the mutation that causes the disease). Finally, the nuclear DNA from the mother and father were injected into the donor egg with the healthy mitochondria—hence the “three-parent baby” label in the headlines.
Why this Breakthrough Matters
Image credits: Andriy Bezuglov | Adobe Stock
This marks a precedent-setting moment in the history of human reproduction. After decades of development, mitochondrial replacement therapy offers a potential end to the suffering caused by mitochondrial diseases, which are often fatal and currently incurable, giving affected families a chance to break the cycle. These births also serve as a powerful proof of principle, demonstrating that complex genetic interventions can be both safe and effective—as far as we know.
Interestingly, the UK’s transparent, carefully regulated approach to approving mitochondrial replacement therapy provides a model for how we might responsibly navigate controversial biotech in the future. Perhaps most significantly, this development sits at the intersection of IVF, gene editing, and reproductive rights, and will shape how we think about biological parenthood, genetic inheritance, and the very boundaries of medical intervention.
What Else Should We Be Asking?
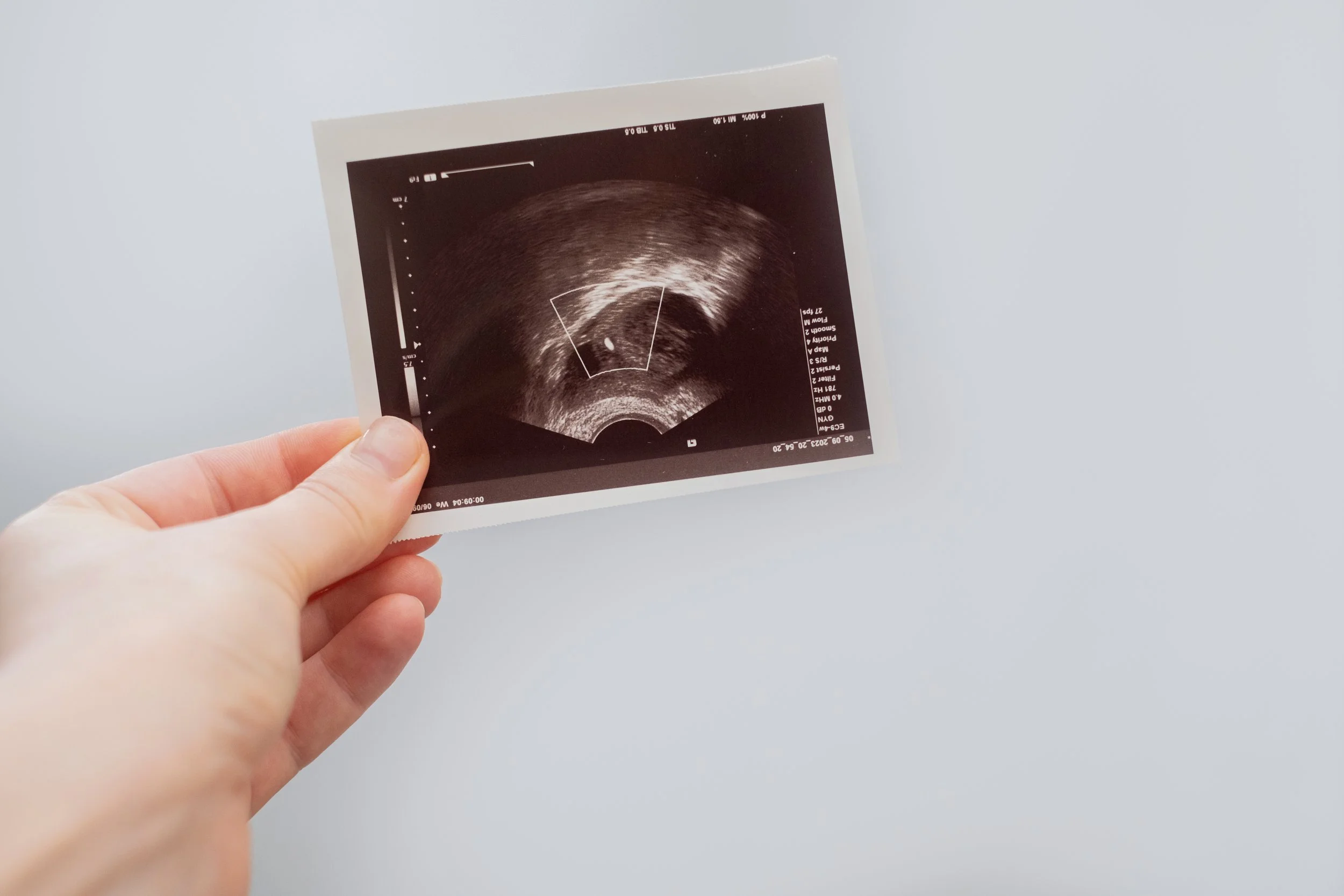
Ultrasound results through IVF | Image credits: Svetlana Golovco | Adobe Stock
This biomedical strategy avoids some of the most contentious dilemmas in genetic engineering, but not all.
One major concern is the potential slippery slope toward designer babies. While mitochondrial replacement therapy is intended (and was used) to prevent disease, some people fear it could open the door to editing DNA for traits like intelligence or appearance. The Pew Research Center reported in late 2020 that majorities of the populations that they surveyed said that “it would be appropriate to change a baby’s genetic characteristics to treat a serious disease the baby would have at birth (median of 70%)”, and a median of 60% responded that it would be appropriate to use genetic techniques to lower the risk of a serious disease appearing later in life. However, in the same surveys, few people (median 14%) said that it would be appropriate to change DNA in order to make a baby more intelligent.
In other words: preventing disease, acceptable. Making enhancements, not acceptable.
Note that no editing of these 8 children took place. In this case, DNA was moved around, but it wasn’t changed in order to reduce or eliminate their risk of devastating mitochondrial disease. The slope to designer babies might be slippery, but it’s likely a longer road from this use case to nightmare sci-fi scenarios.
Another issue is consent and identity. These children carry DNA from a third person who isn’t recognized as a legal or social parent, raising questions about how that knowledge might shape their sense of self and what they have a right to know. Equity is also a concern, as access to advanced reproductive technologies like this often mirrors and reinforces existing global health disparities.
Finally, although the early results are encouraging, we simply don’t yet know how these children will fare long term or how the donated mitochondrial DNA will affect future generations. Again, I’m being careful with my words. Based on what we know today about human genetics, the odds of negative unintended consequences seem to be far, far lower than the almost certain and horrible impacts of the diseases that these families sought to avoid for their children.
What New Futures Become Possible?
Image credits: Ratanachat | Adobe Stock
As I read the news articles about these 8 children, I find myself wondering whether we’re standing at the edge of something profound. If mitochondrial replacement therapy becomes widely adopted, then we could see the virtual eradication of devastating mitochondrial diseases within a generation … at least for those with access to reproductive technologies.
But the implications may stretch far beyond health. As we begin to untangle biological inheritance from the traditional two-parent model, new conversations will emerge about family, identity, and what it means to belong. Mitochondrial replacement therapy may set the stage for broader societal acceptance—or fierce resistance—to more advanced forms of genome editing, such as CRISPR-based germline modifications. And as biotech continues to integrate more deeply into reproduction, we may find ourselves in a future where IVF is no longer the exception but the norm, raising urgent questions about who gets to decide how and what kind of human life is passed on.
At the end of the day, I’m focused on the positive futures that these children and their families likely have in store. As reported by the Guardian, the mother of one of the babies said, “As parents, all we ever wanted was to give our child a healthy start in life. After years of uncertainty this treatment gave us hope – and then it gave us our baby … we’re overwhelmed with gratitude. Science gave us a chance.”
Deeper Conversations Ahead
What do you think?
If science can stop a child from inheriting a terrible disease, should we always use it?
Would knowing you had DNA from three people change how you see yourself?
As technology changes how babies are made, what does it mean to be a parent?
How do we make sure powerful new tools like this are used to help as many people as possible?
These are big questions, and I’d love to hear your thoughts! Send me a message, share this article with a friend, or start a conversation in your community. The future isn’t just something that happens to us. It’s something we get to build, together.
About Tiffany
Dr. Tiffany Vora speaks, writes, and advises on how to harness technology to build the best possible future(s). She is an expert in biotech, health, & innovation.
For a full list of topics and collaboration opportunities, visit Tiffany’s Work Together webpage.
Get bio-inspiration and future-focused insights straight to your inbox by subscribing to her newsletter, Be Voracious. And be sure to follow Tiffany on LinkedIn, Instagram, Youtube, and X for conversations on building a better future.
Buy Tiffany a Cup of Coffee | Image credits: Irene Kredenets via Unsplash.
Donate = Impact
If this article sparked curiosity, inspired reflection, or made you smile, consider buying Tiffany a cup of coffee!
Your support will:
Spread your positive impact around the world
Empower Tiffany to protect time for impact-focused projects
Support her travel for pro bono events with students & nonprofits
Purchase carbon offsets for her travel
Create a legacy of sustainability with like-minded changemakers!
Join Tiffany on her mission by contributing through her Buy Me a Coffee page.